What We Do
We work with clients and academic experts to problem-solve complex questions around drug value and access.
Our work spans a wide range of therapeutic areas including:
- Rare disease
- Gene therapy
- Oncology
- Cardiovascular
- Neurology
- Neuromuscular
- Dermatology
- Infectious disease
- Respiratory

Our Capabilities
With data and evidence at the heart of our work, we combine sophisticated algorithms with intuitive design to:
Characterize the burden of illness, including its key drivers
Understand how the burden of illness changes over time and with new therapeutics
Create visualizations to understand and contextualize the costs of different medications
Determine cost-effectiveness and budget impact of drugs across populations and geographies
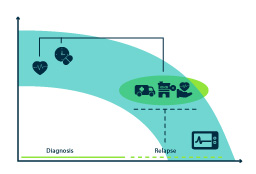
Describe disease trajectories and build epidemiologic models
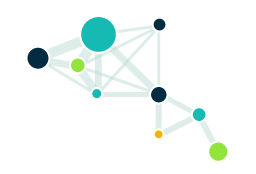
Conduct evidence synthesis of observational and clinical studies
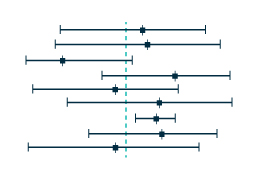
Compare the safety and efficacy of novel and existing treatments

Execute strategic planning for evidence generation, especially for small companies, novel and early therapeutics

Support rapid HEOR response, e.g. for ICER and regulatory groups
Health Economics
Leveraging clinical, epidemiological and economic data to create math-based intuitive models
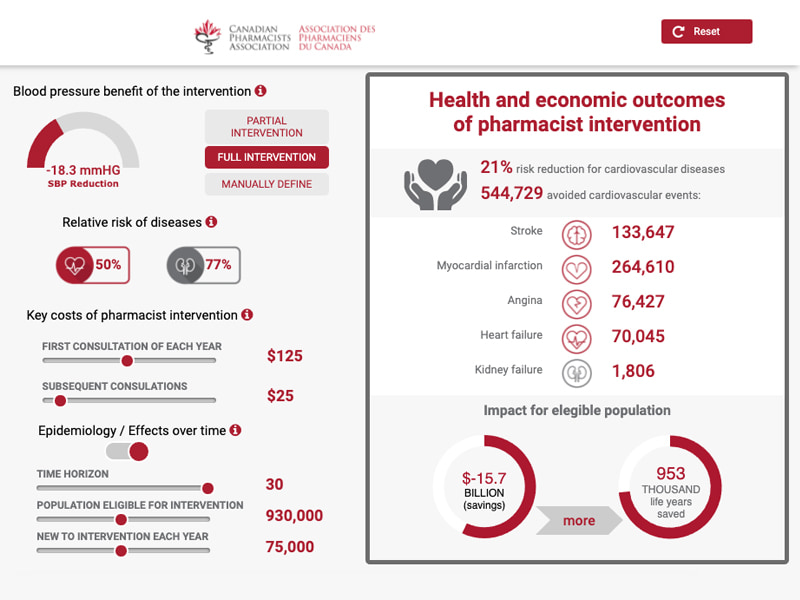
Our approach to developing HEOR models begins by collaborating with clients to design and implement the evidence generation strategy to fill the gaps needed for a customized model.
Our work includes
Pharmacoeconomic and budget impact models for HTA submission (NICE, SMC, CADTH) and peer-review publication
Adaptation of global/core models for local submissions
Risk-benefit and multi-criteria decision models to identify and characterize intended and unintended medication effects
Burden of illness models that synthesize multiple data sources
Early-stage models to guide Phase I/II product priorities
Evidence Synthesis
Assembling relevant pieces of information from multiple sources to address important research questions
We conduct rigorous systematic literature reviews to inform quantitative or network meta-analysis, as well as more descriptive reviews to bring the proof of scientific literature to research areas.
Of course, we can also provide targeted literature reviews where a more efficient strategy addresses a client’s needs.
Reviews are conducted and reported in accordance with recommended guidelines (i.e., Cochrane Collaborative, PRISMA).
Following the review process, our team designs and implements state-of-the-art evidence syntheses, including indirect treatment comparisons.
We look at complex networks, such as those informed by:
Single-arm trials
Observational studies
Individual patient data for a subset of comparators
Systematic reviews of burden of illness data, or natural history evidence to inform economic models for publication
Observational Studies
Bringing a trained eye to look at the clinical landscape and burden of disease for a drug
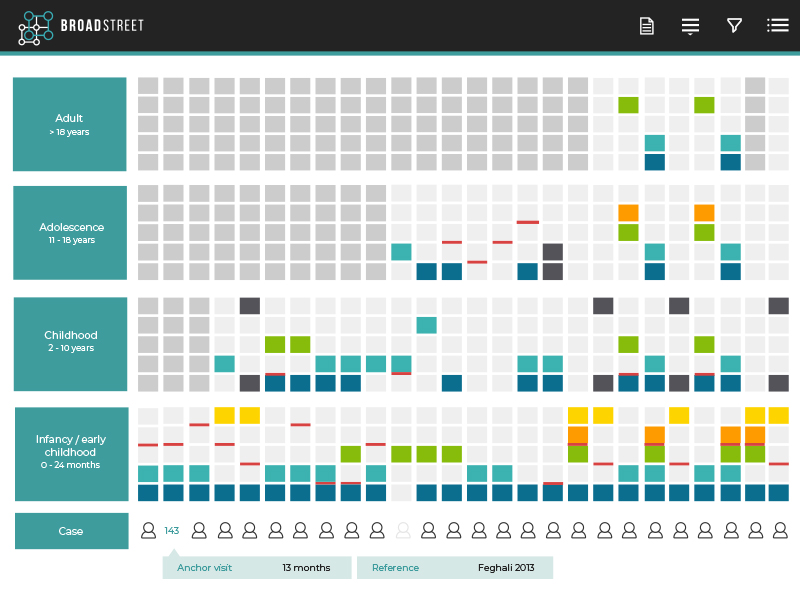
We design and conduct observational studies across a range of epidemiological territories, including estimating treatment effectiveness or safety, characterizing treatment patterns or the burden of disease, and providing parameter inputs for economic models.
Our competencies include standard and specialized methods of study design, and of course, consideration of the most appropriate ways to account for bias.
Retrospective chart review studies
Analysis of existing databases with secure processes for data storage and management
Quantitative/qualitative surveys of patients, healthcare practitioners and/or general public
Prospective observational studies of clinic- or community-based samples to collect select epidemiological, economic and clinician- and patient-reported outcomes (ClinROs and PROs)
We have extensive knowledge of and access to real world data and evidence.
All observational studies include a study design component where we translate a client’s objectives into a feasible study.
We also offer “stand-alone” study design and feasibility including:
Contributing guidance to large multinational studies including disease registries
Contributing guidance to large multinational studies including disease registries
Study protocol to provide comprehensive assessments of timelines,
budget, strengths and limitations before a study is initiated
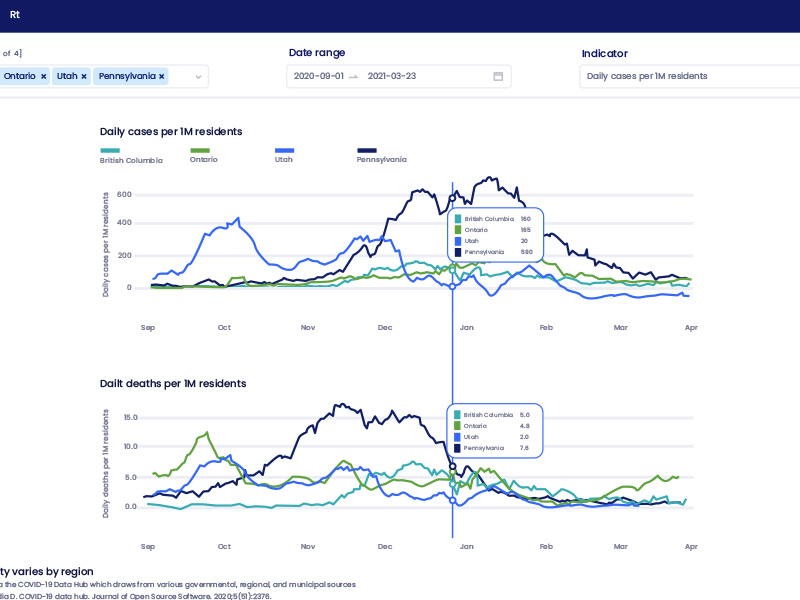
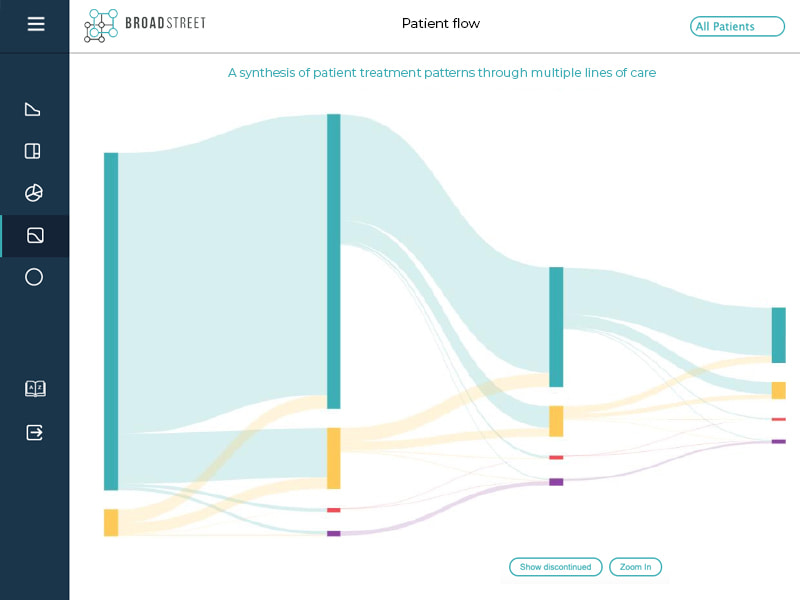
Data Visualization
Translating complex information into simple interactive dashboards that engage users and communicate clear messages
We design strategic visual explanations of sophisticated data that tell a deeper story than possible with one-dimensional number tables, or sets of number-based comparisons.
Every solution is customized
Our goal is to allow users to understand multi-layered concepts and findings at a glance.
Our data visualization work is a way to communicate the evidence we generate – not just an output of that evidence. Every project is different, so every solution is customized.
We don’t rely on a proprietary or off-the-shelf tool.
Many of our visualizations are predictive models that enable users to explore different scenarios by changing 1 or more variables to illustrate the impact those changes have on other factors.
These include:
Value and cost of HCP or drug interventions over time
Budget or pricing impact of drugs across populations and geographies
Financial consequences of adopting a new therapy
Impact of variable factors such as drug adherence against disease trajectories
How can we help you?
