Spinocerebellar ataxias (SCA) are rare inherited neurodegenerative disorders marked by increasing issues with gait, balance, limb coordination, and speech. At present, there is no scale available for measuring the progression of the disease and benefits gained from treatment that covers all the varied aspects of living with SCA, or that is sensitive enough to detect clinical decline in early-stage disease when progression is slow.
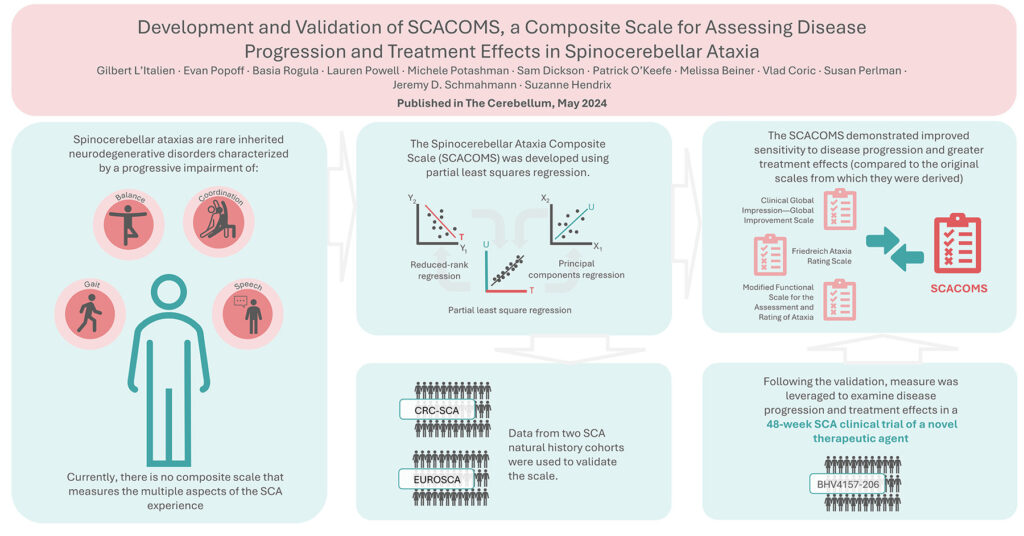
Working with colleagues at Biohaven, University of California Los Angeles, Massachusetts General Hospital, and Pentara, we have developed the Spinocerebellar Ataxia Composite Scale (SCACOMS) using a methodology known as partial least squares (PLS) regression. Published this month in The Cerebellum, this technique is interesting because it allows you to choose and weigh measures objectively, based on how accurately they reflect disease progression, which is a key requirement when a scale is being validated. PLS regression is derived from two other techniques: principal components regression and reduced rank regression. These regression models balance explanatory predictor variation with explanatory response variation; as a result, the correlation between the composite variables and the response variable is greater than with principal components regression, and the method will identify composites with high responsiveness and low bias.
To develop the SCACOMS, data from natural history studies were used, CRC-SCA and EUROSCA. The data from these sources allowed for cross-validation of the scale. Following the validation, SCARCOMS was leveraged to examine disease progression and treatment effects in a 48-week SCA clinical trial cohort (BHV4157-206) exploring a novel therapeutic agent. Items from the Clinical Global Impression—Global Improvement Scale (CGI-I), the Friedreich Ataxia Rating Scale (FARS) – functional stage, and the Modified Functional Scale for the Assessment and Rating of Ataxia (f-SARA) were selected and weighted based on their sensitivity to clinical decline in the natural history studies. The SCACOMS demonstrated improved sensitivity to disease progression and greater treatment effects (compared to the original scales from which they were derived), when applied to clinical trial data. Using the trial data and SCACOMS we were also able to estimate the timing of SCA disease progression.
This work has shown the SCACOMS to be a useful composite measure, effectively capturing disease progression and highlighting treatment effects in patients with SCA. We expect SCACOMS to be a powerful tool in future studies given its sensitivity to clinical decline and ability to detect a meaningful clinical impact of disease-modifying treatments and we hope the measure will contribute to improved outcomes for patients.
You can read more about the SCACOMS measure in the latest issue of The Cerebellum and if you are looking for expert advice on the choice and development of the outcome measures that will achieve your research goals, get in touch to find out how Broadstreet can help.

